AI + STRUCTURED CONTENT: THE PLATFORM FOR PHARMA'S FUTURE
The most efficient way to create, review, update, and publish
Life Sciences documentation
AI + structured content for life sciences
Docuvera’s AI + structured content solution delivers the perfect blend of automation for content development with improved governance, consistency, and control.
Docuvera enables the reuse of approved, compliant blocks of content within and across clinical, regulatory, medical, safety, CMC, and other documents. Author, review, and update once to reuse across multiple documents. Docuvera’s automated, simple user experience provides a seamless transition for medical authors upgrading from desktop publishing software.
A powerful and easy-to-use service in one intuitive platform
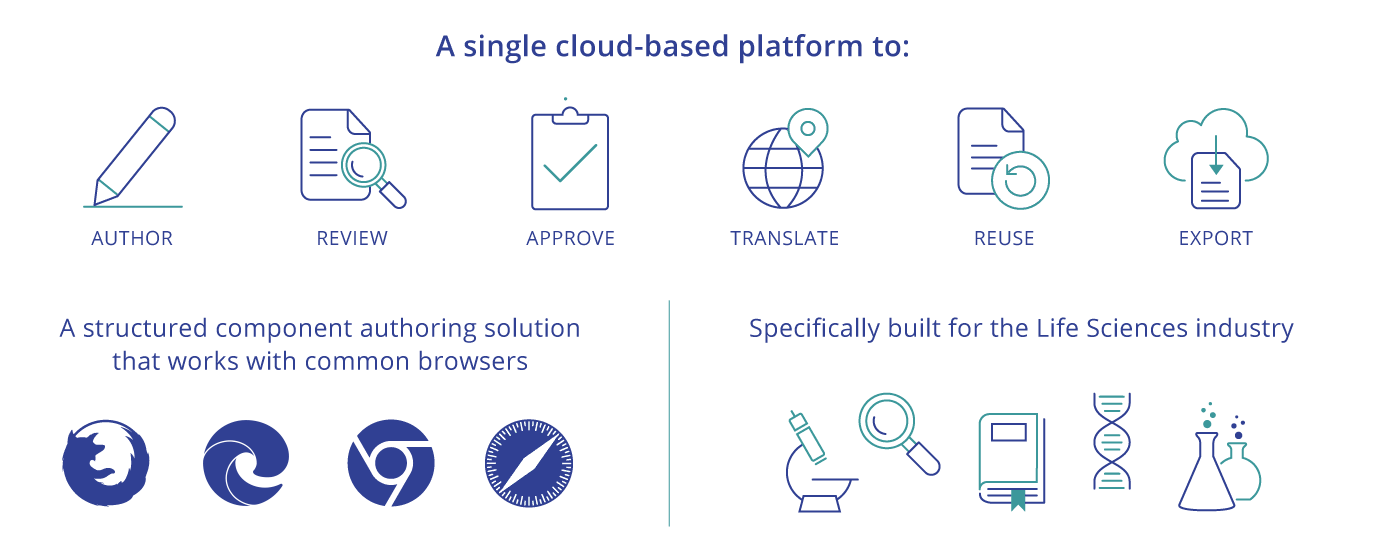
The Docuvera platform combines an intuitive web interface with the latest cloud and AI technologies to provide a powerful yet easy-to-use service that relieves the pain of traditional document creation.
A single platform to author, review, approve, translate, and publish regulated documents by reusing approved, compliant component content
Component management made simple
Authors can easily reuse approved blocks of content to assemble documents. No technical expertise or expensive support required.
Intelligent component reuse
When your content is created as structured components, it can be reused across multiple documents, by as many authors and contributors as needed. No more wasted time continuously copying and pasting or creating content from scratch.
Seamless propagation of modified content
Docuvera notifies authors immediately when source content changes and gives them the opportunity to review and accept those changes into their documents. There are no delays or confusing back-and-forth between content developers.
Collaborative web review and approval
Cross-department review of documents can happen collaboratively in a single forum for discussions and resolutions. No more manual merging of Word track changes.
Omni-channel document publishing from the same project
Create Word, PDF, HTML and other documents from the same project by applying different styles during the publishing process. No need to manually create different document types.
How does Docuvera work?
- The simple, drag-and-drop interface allows authors to easily reuse pre-approved component content to assemble new documents. And, our auto propagation feature can create documents automatically from existing component content.
- Collaborative workflows allow users to simultaneously contribute to authoring, review, and approval of documents.
- Word, PDF, HTML, and other document types are published from the same project, but each has a specific set of styles and layout.
- Changes to reused component content seamlessly propagate to documents where information is reused. Local authors can review source changes and incorporate updates as necessary.
- Our system stores a clear audit trail of document and component modifications, workflows, and published documents for compliance and quality purposes.
Authoring, collaboration, reuse and publishingright at your fingertips.
Long gone are the days of time wasted on locating and updating multiple documents stored in silos. With Author-it, any object – be it a word, phrase, or paragraph – can easily be changed in one place and pushed out to multiple publishing outputs simultaneously. No more copy and paste!

Docuvera solutions
Harness AI + structured content for modern pharma documentation.
Clinical
Docuvera brings efficient and easy-to-use component-based authoring capabilities to the creation of and revision process for pre-clinical and clinical content.
Global Labeling
Docuvera’s end-to-end solution reduces labeling content production times, ensures alignment to compliant content, and creates faster time-to-market for regulatory submissions.
Medical Information
Docuvera reduces medinfo content production times and enables omnichannel distribution of medical content to Health Care Professionals.
Chemistry Manufacturing and Controls
Docuvera enables the efficient creation and maintenance of CMC documentation by reusing core component content to assemble local documentation.
Chemistry Manufacturing and Controls
Docuvera enables the efficient creation and maintenance of CMC documentation by reusing core component content to assemble local documentation.
Aggregate Reporting and Safety Writing
Docuvera brings component-based reuse to the creation and maintenance of aggregate safety reports for all stages of the product lifecycle.
Quality Documents/SOPs
Review, approve, translate, reuse and publish quality documents and reuse approved content components to efficiently assemble accurate SOPs with Docuvera.
“Docuvera is a growing, innovative technology. I really appreciate the team’s responsiveness to our needs. Migration of existing content into Docuvera is a big piece for the adoption, and it helps the authors adopt the solution when they see their content in the new technology.”
Cecil Lee,
Lilly
Learn how Docuvera will revolutionize your content capabilities
Get more information or request a demo.